In an early phase 1 clinical trial a new drug for primary mitochondrial myopathies, KL1333, has been found to be safe and potentially improve muscle symptoms in people with the condition. Researchers are now looking to move KL1333 on to the next stage of development to confirm it is beneficial.
New drug in development for primary mitochondrial myopathies

Primary mitochondrial disease (PMD) is a rare condition affecting mitochondria. Mitochondria are small compartments within cells that produce the energy our bodies need to function. They act a bit like the battery of the cell and are found in muscle cells – where energy use is very high. In PMD, there are changes to mitochondria which disrupts how energy is made, often leading to fatigue and muscle weakness.
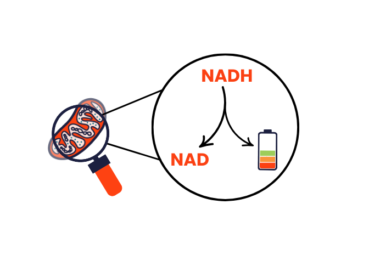
Mitochondria rely on a small molecule called nicotinamide adenine dinucleotide (NAD), which exists in two forms – NAD and NADH.
NADH helps make the molecule that provides energy to the cells – the cell’s ‘energy currency’. Everything that requires energy has an energy currency. For example, when we charge a phone or laptop battery, the energy currency is electricity. Once this energy is made, NADH transforms into NAD. The balance between NADH and NAD levels is important for cells to produce energy properly.
In many mitochondrial myopathies, there are changes to mitochondria that cause it to produce more NADH than NAD. This disrupts energy production.
A potential new drug for primary mitochondrial myopathies
Treatment options for PMD are limited, so researchers are looking to find more options. Dr Pizzamiglio, from University College London, and colleagues have identified a drug called KL1333 that may be more effective than current treatment options.
KL1333 is designed to improve muscle strength by increasing NAD levels in mitochondria. It’s thought to react directly with NADH to transform it into NAD and promote the function of a protein that converts NADH to NAD. Through both these actions, NAD levels should increase in mitochondria, increasing energy production and muscle strength.
Improving drug development for rare diseases like primary mitochondrial myopathies
Developing drugs for people with rare diseases like PMD can be very difficult. There are few people with the condition, making it hard to gather lots of information about how the condition progresses and the impact a new treatment could have. Researchers need to know a treatment works as expected in the body. But more importantly they need to know it is having a beneficial impact on symptoms and progression.
Dr Pizzamiglio and colleagues worked with people living with PMD to understand what a meaningful effect from a treatment would be. People living with PMD highlighted that fatigue and muscle weakness were the most difficult symptoms and treatments that target these symptoms would be valuable.
Phase 1 clinical trial results for the new potential treatment
To check whether KL1333 could work as a treatment for people with PMD, researchers completed a clinical trial. Clinical trials test if a new drug is effective and safe. Typically, volunteers without a health condition are recruited in a phase 1 clinical trial. Recruiting people without a condition is often quicker and easier to do. This allows researchers to quickly gather more evidence about the treatment before moving to the next stage. In this phase 1 trial, the researchers recruited both people with and without PMD. Overall, they recruited 72 people, including 64 without the condition and 8 with PMD. This way the researchers could gather enough safety data but also get ahead on collecting evidence that the drug may be beneficial for people with PMD.
54 participants received KL1333 and 18 received the placebo (dummy drug). Out of these participants six with PMD received KL1333 and two with PMD received the placebo. They were also given different dosages of KL1333 to see how these affected people with PMD.
KL1333 was well tolerated, meaning there wasn’t many side effects. Some people did experience mild intestinal irritation like diarrhoea and bloating. However, these side effects were reduced if the dose was reduced. The treatment also showed signs that it may improve muscle symptoms, when measuring using the 30 second Sit to Stand Test. Participants who received KL1333 were able to complete more full stands from a seating position in 30 seconds, compared to those who received the placebo. People who received KL1333 also reported less fatigue.
Next steps
Overall, KL1333 was found to be safe and potentially improves muscle strength for people with PMD. However, as KL1333 was only tested in a very small number of people with PMD, a larger and longer clinical trial is now needed to confirm if it is beneficial.
This research is moving in the right direction, but there is still a long way to go before KL1333 could become a treatment for PMD.


