Early laboratory studies have shown a small molecule called MZ-101 can reduce the harmful buildup of glycogen found in the muscle cells of people living with Pompe disease. Evidence so far suggests that combining the use of this molecule and existing enzyme replacement therapy (ERT) may be a more effective way of treating the condition.
A potential treatment for Pompe disease shows future promise

What is Pompe disease?
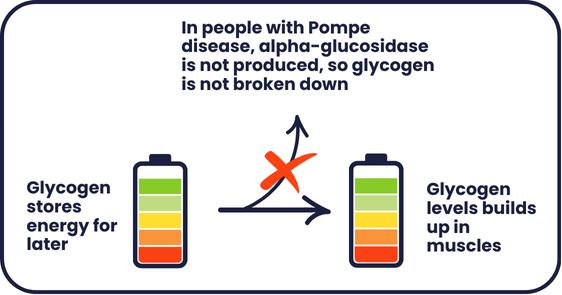
There are two main types of Pompe disease – also known as acid maltase deficiency or glycogen storage disease type II. In Infantile-onset Pompe disease (IOPD), symptoms begin at a few months old, while in late onset Pompe disease (LOPD), symptoms appear in late childhood/adolescence/adulthood and are often less severe. Both types are caused by changes in the GAA gene. The GAA gene provides the instructions to make a molecule called alpha-glucosidase, which is involved in releasing energy to cells.
We need energy to keep our body functioning as it should. We get energy from food, which is stored in the body as glycogen until it’s needed. This is like storing energy in a battery. Alpha-glucosidase helps break down this glycogen into glucose, releasing energy to cells.
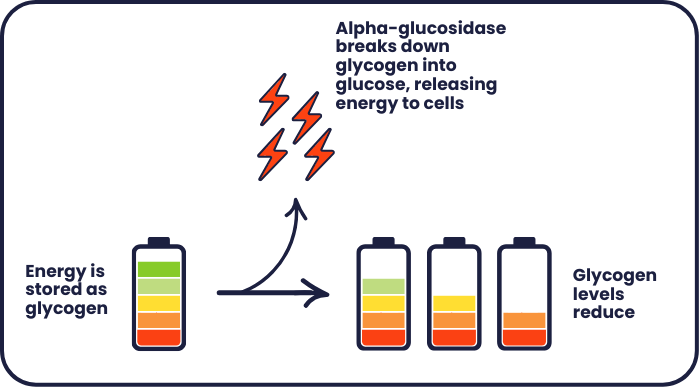
In people living with Pompe disease, changes in the GAA gene mean that less alpha-glucosidase is produced. Glycogen builds up in muscle tissue, causing damage to the cells and muscle weakness. This is a bit like overcharging a battery – causing it to overheat and not perform as well as it should.
Current treatments for Pompe disease
Current treatments for Pompe disease include treatments which mimic alpha-glucosidase. They reduce the build-up of glycogen by breaking it down to release glucose. These types of treatment are known as enzyme replacement therapy (ERT). Unfortunately, not all people with Pompe disease find ERT effective. It can also be tiring to receive as it’s administered by frequent slow injections into the bloodstream, which can impact on a person’s quality of life. Researchers are working hard to find new treatment options which are more effective and help improve quality of life of people with the condition.
How does MZ-101 work?
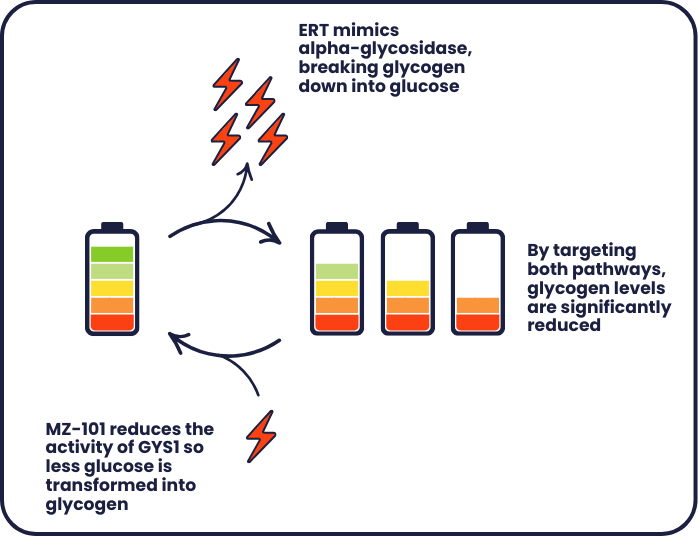
In early laboratory studies, a small molecule called MZ-101 reduced the amount of glycogen in muscles. Instead of targeting alpha-glucosidase, MZ-101 reduces the activity of a different molecule called glycogen synthase (GYS1), which helps to store energy by turning it from glucose (which we get from our food) into glycogen.
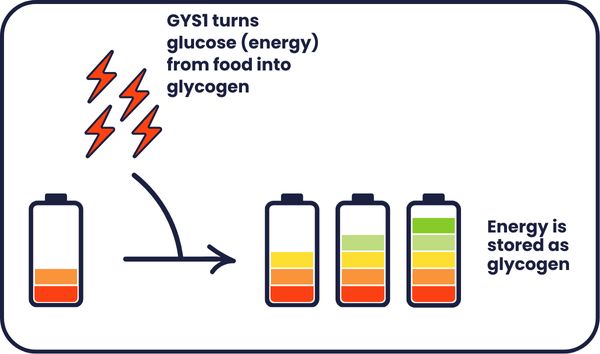
Reducing the activity of GYS1 causes cells to produce less glycogen, which prevents glycogen build up in the first place. To test if MZ-101 does reduce glycogen levels, researchers used models which mimic the condition. These models help them study what happens in the condition and determine whether the damage can be corrected. Models can include animals, as well as cells grown in a dish
When using these models, researchers found that treatment with MZ-101 reduced levels of glycogen. However, glycogen levels did not reduce to levels seen in a model which didn’t have the condition (known as a control). Researchers then decided to try combining MZ-101 with the existing treatment (an ERT) to see if that would lead to a greater reduction. In both cell and mouse models, a combination of the treatments was more effective at reducing glycogen levels compared to either treatment alone.
MZ-101 and a phase 1 clinical trial
Following the above findings, MZ-101 was tested in a phase 1 clinical trial to check it’s safe. The trial recruited participants without a health condition, which is common for a phase 1 trial. MZ-101 was found to be well-tolerated and early findings showed glycogen levels were reduced. This shows the treatment is doing what it’s designed to do in the body. However, further research is needed to see if this has a beneficial effect in people with Pompe disease. The combination of both MZ-101 and an ERT also still needs to be tested in people.
Next steps
The results from the phase 1 trial are promising, but MZ-101 now needs to be tested in people with Pompe disease. Researchers are working towards starting a phase 2 trial. This will gather the evidence needed to see if the treatment is safe and effective. Developing treatments can take a very long time. It takes roughly seven or eight years for a treatment to be approved for use after a phase 1 trial. While the results so far are promising, there is still a long way to go before MZ-101 could become a treatment for Pompe disease.


