Muscle wasting and weakening conditions can be caused by loss of the DNA code which makes certain large proteins that muscles need to work properly. Gene therapies try to deliver this code back into the muscle, but the size of the DNA code can make this difficult. Researchers have found a new way to deliver the code in parts that get joined together inside the muscle cells. This could lead to new gene therapies to treat muscle wasting and weakening conditions.
Researchers develop a new approach for muscle gene therapies
The size challenge in gene therapy
Gene therapy has been developed for several different muscle wasting and weakening conditions, but efforts are ongoing to make it work more effectively. One of the challenges is that some of the proteins that muscle cells need are very large. The instructions to make a protein are contained in the DNA code of a gene, and the length of this code is measured in chemical blocks called bases. The larger the protein, the longer the sequence of DNA bases needed. For example, the coding sequence for the dystrophin protein, the loss of which causes Duchenne muscular dystrophy, is more than ten thousand bases long – the coding sequences of most genes are around just one thousand bases. Another example is the dysferlin protein, the loss of which causes a type of limb girdle muscular dystrophy and some other muscular dystrophies. Its coding sequence is over six thousand bases.
The challenge of using empty shells of viruses
The best way that researchers have so far found to get coding sequences into the body’s cells is to use the empty shells of viruses. When a virus infects a cell, it uses these shells, called capsids, to package up its own genetic code and send it off to infect other cells. They can be made artificially in laboratories, without the harmful viral code, and used instead to deliver gene therapies. The capsids that currently work best for gene therapy are from a virus called adeno-associated virus, or AAV. AAV is good at getting to lots of our cells without triggering the body’s immune defences.
However, the coding sequences of dystrophin and dysferlin are physically too large for the AAV capsid. At just 26 nanometres wide (a thousand of them would fit across a human hair), it can hold a sequence only as long as the virus’s own genetic code, which is less than five thousand bases.
Ribozymes as a possible solution
Recent research has found a new way around this. In the last few decades, a type of molecule called a ribozyme has been discovered and studied. Ribozymes are sequences of RNA (a copy of DNA used to make proteins) that can cut themselves in two. In doing so, they leave behind specific chemical markings on the new ends that have just been cut. Researchers in New York and Massachusetts noticed that certain proteins in the cell are able to recognise those markings and stitch the ends back together. They thought this could be used to get the cell to join up the ends of two different RNA molecules, such as two parts of a gene therapy. They called the approach StitchR, for Stitch RNA.
To test StitchR, the researchers made one RNA molecule consisting of the first part of the dysferlin coding sequence followed by a second ribozyme and a RNA molecule consisting of a ribozyme followed by the other part of the dysferlin coding sequence. They filled some AAV capsids with one molecule and other AAV capsids with the other molecule and injected them together into a mouse model of limb girdle muscular dystrophy.
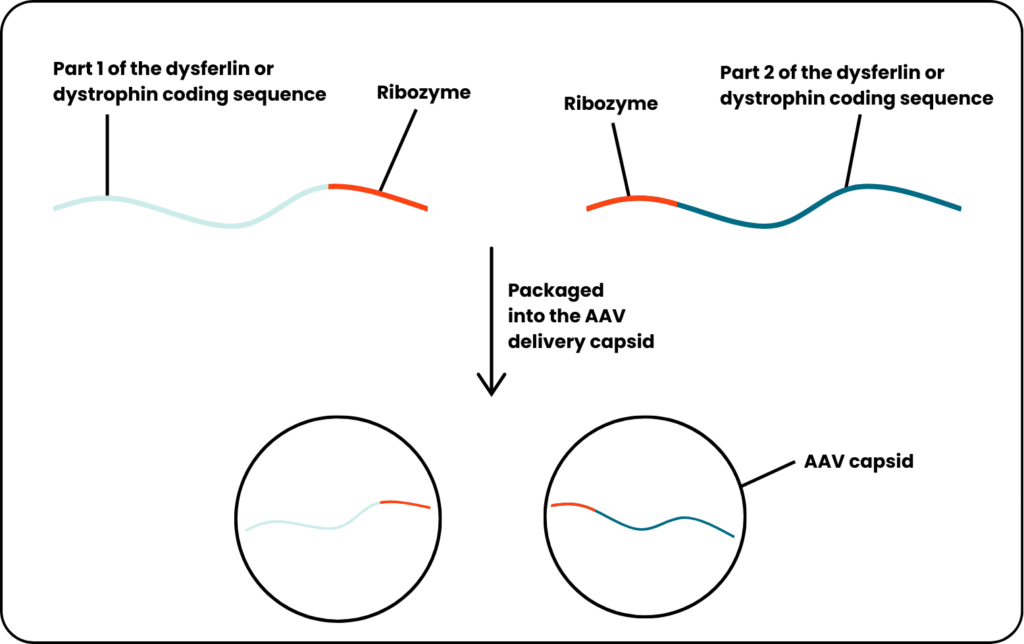
Once inside the mouse muscle cells, the ribozymes are activated and cut their respective molecules. The cell’s repair mechanisms, recognising the markings of the cuts, joins together the two parts, resulting in the restoration of dysferlin protein to the mouse’s muscles.
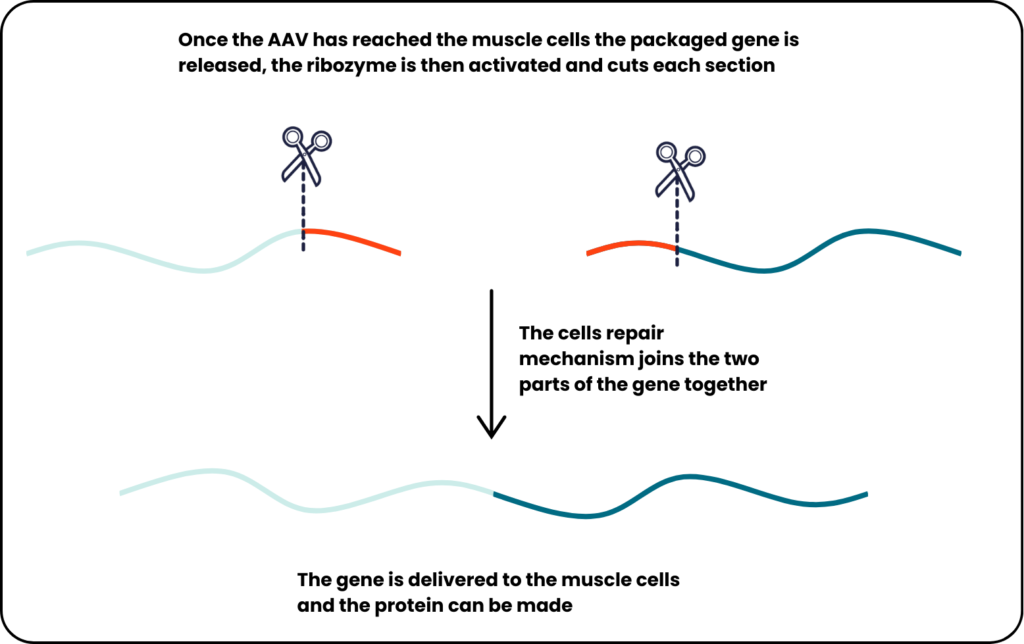
The researchers also tried this approach with dystrophin. For this, they delivered the coding sequence for a slightly shortened but still functional protein, since even cut in two the dystrophin coding sequence is still too big for the AAV capsid. The approach led to improvements in several measures of the condition in the muscles of the Duchenne muscular dystrophy mouse model.
Significance
Approaches that join up coding sequences of large genes inside the target cell have been in development for many years as potential treatments for Duchenne muscular dystrophy, the dysferlinopathies, and other diseases, but unfortunately, they have struggled to work effectively. This new method may overcome some of the challenges, and the results in mouse models are encouraging. However, further testing will be needed to know if this research can lead to new medicines.


