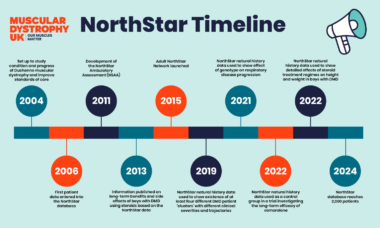
Since the programme first began in 2004, it has reached significant milestones. From improving the design of the clinical trials, to improving quality of life and the management of DMD, the NorthStar programme has made a real impact for people living with DMD.
The NorthStar Ambulatory Assessment
One of the most significant achievements from the NorthStar clinical network is the development of the NorthStar Ambulatory Assessment (NSAA). A rating scale which uses 17 tests that only take 10 minutes to complete and assess the abilities of boys with DMD not using a wheelchair. The NSAA is now widely used to track of the progression of DMD. It is also used in clinical trials to help assess the benefits of treatments.
In 2016 researchers from the NorthStar clinical network published a study using the NSAA to detail the ‘natural history’ of DMD − how the condition normally changes over time. This information has helped to design clinical trials, providing a ‘baseline’ against which researchers can measure changes in the condition.
It could also help to reduce the number of boys who receive the placebo (dummy drug) in a clinical trial. Placebo groups are vital in clinical trials. They make sure any potential benefits from a treatment are due to the treatment and not any other factors. Reducing the number of boys who receive the placebo could make it quicker for clinical trials to get started and determine if a treatment is beneficial.
Steroids for treating Duchenne
Data from the NorthStar natural history study played a key role in a 2013 research study which helped convince clinics across the country to use steroids (glucocorticoid) to manage DMD. The study provided detailed information on the benefits and side effects of steroids, highlighting the real-world benefits of steroid treatment. As a result, children with DMD are now walking for around 3.5 years longer than in the previous decade.
Researchers have since used data from the NorthStar natural history study to understand the long-term impact of continuous steroid use. Dr Federica Trucco and colleagues looked at the impact of steroids on heart and lung health. They found that steroids delayed the need for breathing support (like a facemask) and the onset of heart problems.
On the other hand, Dr Shuko Joseph and colleagues found that steroids increase the chances of bone fractures. They also found that different steroids affect that risk by different amounts. This could lead to improved care for people with DMD by finding ways to improve bone health.
More recently, the NorthStar network was involved in a study looking at a drug called Vamorolone – an alternative to steroids. In 2024, Vamorolone was recommend by the National Institute for Health and Care Excellence (NICE) as an option for treatment DMD.
Mapping Duchenne muscular dystrophy condition progression
Clinicians and parents have known for a long time that every boy with Duchenne is different. The progression of the condition can vary from person to person. NorthStar network researchers used data from 395 boys from the natural history study to map out how the condition changes over time. They grouped the changes of these boys into four clusters, depending on the severity of their disease and how rapidly their condition progresses. This information will help doctors provide boys with DMD and their families more information about the long-term impact of the condition, and tailor care and support more effectively. It will also help to design clinical trials, and make sure that boys with DMD of all severities are included.
Expanding the NorthStar programme to adults with Duchenne muscular dystrophy
Improvements in care over the past decade mean people with DMD are living longer. But there are fewer clinical trials available for adults than children living with the condition. To address this, NorthStar has expanded to include information from adults. The Adult NorthStar programme aims to establish best-practice guidelines for care and support and to support future clinical trials.
“Thanks to funding from Muscular Dystrophy UK, we have established the Adult NorthStar Network for young men and adults with Duchenne. This will help the monitoring and implementation of optimal standards of care for this group, providing a strong foundation for clinical trial in the future.”
– Professor Ros Quinlivan, University College London Hospital
This has led to the development of the first-ever clinical care guidelines for adults with Duchenne. Improving the standards of care and support for men with DMD will help improve their life expectancy and quality of life.
